Regenerative medicine has taken a significant step thanks to the research conducted by the Terasaki Institute for Biomedical Innovation in Los Angeles. Scientists have developed an innovative bioink that uses a time-release hormone to promote growth and regeneration of 3D printed muscle tissue. The finding could have profound implications for the treatment of patients who have suffered muscle loss or damage from trauma, disease or surgery.
La 3d printing it has already revolutionized several sectors, from industrial production to design, but it is in the field of regenerative medicine that it could have the most profound impact. A bioink (and the ability to create tailor-made tissues and organs for patients) has the potential to transform disease treatment and surgery. One of the main challenges is creating fabrics that not only look like natural ones, but also function like them.
The context of the challenge
Generating muscle tissue similar to the “original” one is not a simple task. Tissue is composed of many different cell types, and the environment surrounding muscles is regulated by complex biochemical and biomechanical pathways. These include inflammatory cytokines and growth factors that maintain internal stability and support tissue repair.
The traditional approach and its limits
Currently, repairing muscle that is damaged or lost due to injury, disease, or surgery involves transferring healthy muscle to the affected site, a technique called autologous transfer. However, this method not only negatively impacts the area from which the healthy tissue is taken, but can hinder the functional recovery of the muscle.
An innovative solution: bio-ink
Bioink developed by the Terasaki Institute for Biomedical Innovation (TIBI) could overcome the limitations of autologous transfer, enhancing 3D printed skeletal muscle builds.
Normal skeletal muscle development is a gradual process. It relies on myoblasts, precursors to muscle cells, that fuse together to form myotubes, which eventually become muscle fibers. This process is called myogenesis. Therefore, in muscle engineering, it is critical that functionality is maintained by ensuring that maturing muscle cells are structurally aligned and that their survival is enhanced.
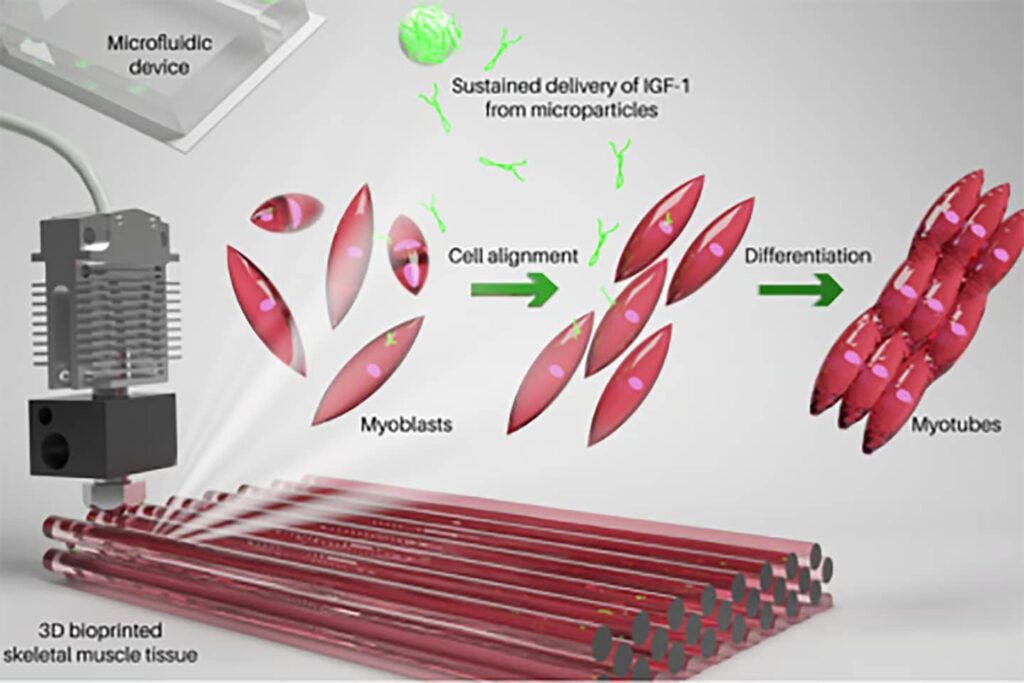
The key ingredient of bioink: IGF-1
To simulate myogenesis, the researchers relied on a key ingredient in their bioink: the growth factor IGF-1. This hormone, with a molecular structure similar to insulin, is essential for the normal growth of bones and tissues.
The bioink is composed of a biocompatible gelatin-based hydrogel called gelatin methacryloyl (GeIMA), myoblast cells, and IGF-1-coated PLGA microparticles designed to slowly release the hormone as the particles degrade.
Promising results
The researchers found that three days after bioprinting the muscle builds the myoblasts were viable, confirming that the printing process had not damaged the cells. They observed improved alignment of myoblasts and fusion of myoblasts to form myotubes. These results were particularly evident in constructs containing IGF-1.
Towards a regenerative future
These results are just the beginning. With further research and development, we may see widespread use of this technology in surgery and regenerative medicine. As he pointed out Ali Khademhosseini, corresponding author of the study, “there is great potential in using this strategy for the therapeutic creation of functional and contractile muscle tissue.”
The research was published in the journal Macromolecular Bioscience, and I link it here.


