Do you know the Zolgensma? It is currently the most expensive drug in the world. A one-time treatment of this drug (life-saving for a child) costs 2 million euros.
Among gene therapies, the one based on Zolgensma treats spinal muscular atrophy, a rare genetic disease that damages nerve cells, leading to muscle decay. Today the exorbitant price of Zolgensma is a value that seems very anomalous, but by the end of the decade there will be dozens of gene therapies which will cost hundreds of thousands to millions of euros for a single dose.
The US FDA alone plans to approve 2025 to 10 gene and cell therapies each year by 20.

Health systems around the world are not equipped to handle these new gene therapies
Currently, only 5% of the roughly 7.000 rare diseases have an approved drug, leaving thousands of conditions without a cure. That won't be the case soon.
In recent years, genetic engineering technology has made great strides towards the ultimate goal of curing diseases by modifying the genetic instructions of a cell (in Italy thanks also at Telethon!). The resulting gene therapies will be able to treat many diseases at the DNA level with a single dose.
Gene therapies have the potential to save many lives and alleviate much suffering, but it's bittersweet news. Well-being is such only if shared: health (and economic!) Systems around the world are not equipped to manage these new therapies. Creative new payment systems will be needed to ensure equal access for all.
A cure for (almost) everything
Thousands of diseases are the result of DNA errors that prevent cells from functioning normally. By directly correcting disease-causing mutations or altering a cell's DNA to provide it with new tools to fight disease, gene therapies offer a powerful new approach to medicine.
There are 1.745 gene therapies in development around the world. A large part of this research focuses on rare genetic diseases, which affect 400 million people around the world.
We may soon see cures for rare diseases likesickle cell anemia, muscular dystrophy , progeria, a rare and progressive genetic disease that causes children to age rapidly. In the future, gene therapies could help treat more common conditions, such as heart disease and chronic pain.
Prices? To the stars
The problem is that these gene therapies will come at a huge price.
Gene therapies are the result of years of research and development totaling hundreds of millions or billions of dollars. Sophisticated manufacturing facilities, highly skilled personnel and complex biological materials distinguish these therapies from other drugs.
Pharmaceutical companies say cost recovery, particularly for drugs with fewer potential patients, means higher prices. And the toll of these sky-high prices on health systems will not be trivial.
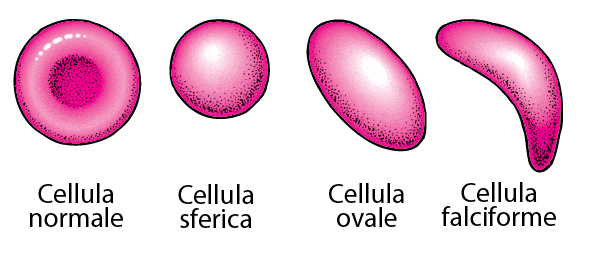
Consider gene therapies for sickle cell disease, which are expected to be available in the next few years. The estimated price of this treatment is 1,7 million euros per patient. Tens of millions more for the healthcare system, even if a minimal percentage of patients were to be treated. And it's just one drug: dozens of similar gene therapies would put a strain on public health systems, and would also create many difficulties for private insurance companies in healthcare models like the US one.
Gene therapies: lower costs, find new ways to pay
One solution to improve patient access to gene therapies would be to simply ask drug makers to charge less for them – a tactic recently adopted in Germany. This comes with risks, however: it could lead to companies simply refusing to provide treatment in certain locations.
I think a more balanced and sustainable approach is twofold. Short term, it will be important to develop new payment methods that entice insurance companies to cover high-cost gene therapies and distribute risks among patients, insurance companies and drug manufacturers. Long-term, improved gene therapy technology will inevitably help reduce costs.
Results-based insurance?
For innovative payment models, a proven approach is to tie the coverage of patient health outcomes. As these therapies are still experimental and relatively new, there isn't much data to help insurers make the risky decision whether to cover them. If an insurance company pays $ 1 million for therapy, it better work. In outcomes-based models, insurers will pay for part of the therapy upfront and the rest only if the patient improves, or they will cover the entire cost upfront and receive reimbursement if the patient does not improve. These models help insurers share financial risk with drug developers.
On the cost front
The key to improving access to gene therapies will be investing in new technologies that simplify medical procedures. Improving access to gene therapies requires collaboration and compromise between governments, nonprofits, pharmaceutical companies and private companies. Taking proactive steps now to develop innovative payment models and invest in new technologies will help ensure health systems are ready to deliver on the promise of gene therapies.