Scientists at Stanford University have developed a chlorine battery that is not only rechargeable, but offers approximately six times the capacity of today's lithium-ion solutions. The breakthrough is based on stabilizing volatile chlorine reactions within the device. It could one day provide the basis for high-performance batteries that power, for example, smartphones for weeks before running out of power.
The new battery is described as an alkali metal chlorine battery and is based on a chemistry that first emerged in the 70s called lithium thionyl chloride. These batteries are highly regarded for their high energy density, but they are not rechargeable. They are based on highly reactive chlorine which makes them unsuitable for multiple uses.
Substantial differences
In a normal rechargeable battery, electrons travel from side to side while discharging and then return to their original shape when the battery is recharged. In this case, however, sodium chloride or lithium chloride is converted to chlorine, which is too reactive to be converted back to chloride with great efficiency.
The authors of this new study may have found a solution to this problem. The team was experimenting with sodium chloride and chlorine to try to improve the performance of this battery, but found that the chemical had indeed stabilized. This provided the battery with some degree of recharging. Subsequent investigation led the team to develop a new porous carbon electrode material that acts like a sponge, absorbing irregular chlorine molecules and safely storing them to be converted back into sodium.
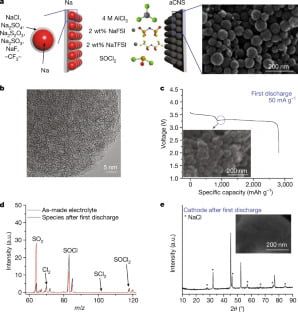
The chlorine molecule is trapped and protected in the tiny pores of the carbon nanospheres when the battery is charged. So when the battery needs to be discharged or discharged. We can discharge the battery and convert the chlorine to produce NaCl – table salt – and repeat this process for many cycles. Currently we can cycle up to 200 times and there is still room for improvement. A well-maintained lithium-ion battery, so to speak, it can fit for 500-1000 cycles.
Guangzhou Zhu, Stanford University
Chlorine battery, high density
Through their experiments, the team also demonstrated a very high energy density for the prototype battery. 1.200 mAh per gram of electrode material, approximately six times that offered by today's lithium-ion battery technology.
The team imagines that the battery could be used in hearing aids or remote controls. It could also power devices that require only infrequent charging (satellites or remote sensors that could be recharged with solar energy). For use in smartphones and electric vehicles, scientists will need to scale up the battery and design a suitable structure. This means first of all increasing the number of times it can be used safely.
Research on the chlorine battery was published in the journal Nature.


