Is called ionocaloric cooling, and it's a new technology that could change the way we cool down, replacing the methods currently used in refrigerators and air conditioners.
“There is no alternative solution that is efficient. Neither 100% safe, nor 100% harmless to the environment,” says the mechanical engineer Drew Lilley from the Lawrence Berkeley National Laboratory in California. “But we think that the ionocaloric cooling cycle, if well developed, can achieve these objectives.” Is he right?
What is ionocaloric cooling?
Ionocaloric cooling works by passing a current through a material that moves the ions within it, changing its melting point and therefore the temperature. A bit like the principle used on winter roads. Do you know? Come on, when you sprinkle salt to lower the melting point of water.
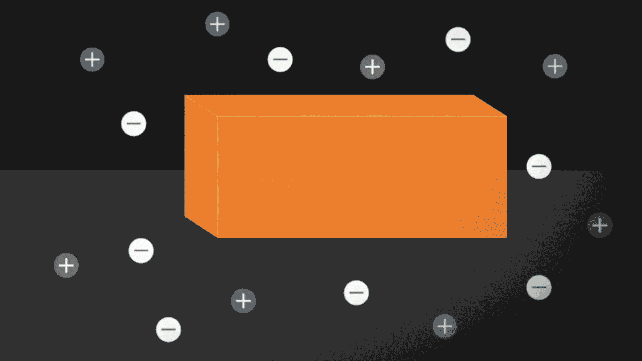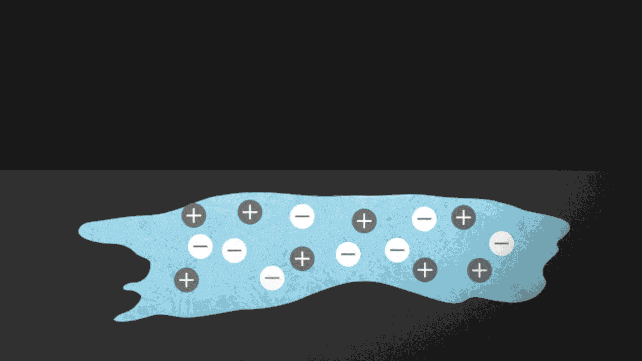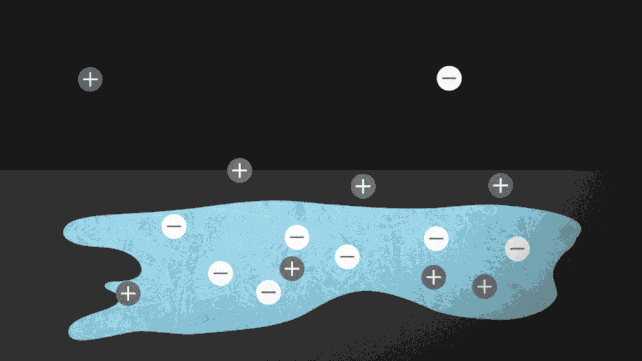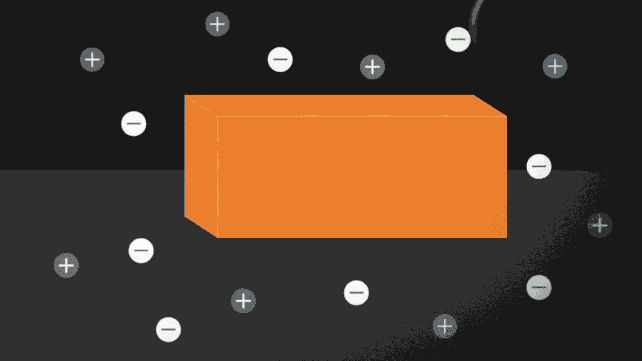
In the paper published in Science (I link it here) the research team conducted experiments using a salt made of iodine and sodium to dissolve ethylene carbonate. This is a common organic solvent also used in lithium-ion batteries (and can be produced from carbon dioxide).
This means that the system not only emits no greenhouse gases, but may even have a global warming potential negative.
Does it really work?
It seems so. Ionocaloric cooling achieved an outstanding result during a recent experiment, in which it was possible to lower the temperature by as much as 25°C (45 degrees Fahrenheit) with less than a single volt of charge. A goal that surpasses that achieved by other caloric technologies.

Further research is underway to evaluate the large-scale application of this technology which uses less (and not harmful) chemistry and more physics to cool.
The opportunity to do without refrigerant gases (which, I remember, by law will be reduced by 79% by 2030) and relying only on ions is too tempting to pass up.
I will keep you posted on the progress!


