The progression of Duchenne muscular dystrophy (DMD) can be delayed in mice by supplementing their diet with urolithin A, according to new results reported today. The results, published on Science Translational Medicine, raise hope that new treatment options may one day be developed for muscular dystrophy, an untreatable genetic condition characterized by progressive muscle degeneration. About 1 in 3.500 children are born with DMD, which significantly reduces life expectancy.
The new research is led by Professor Johan Auwerx, MD, PhD at the Swiss Federal Institute of Technology EPFL, and by the University of Lausanne in collaboration with scientists from the Swiss company Amazentis. He highlights the important role that defective mitochondria can play in muscular dystrophy. The power plants of cells, the mitochondria, produce the energy necessary for normal muscle function. But muscle cells taken from human DMD patients and mice in equivalent conditions show significant defects in mitochondrial activity.
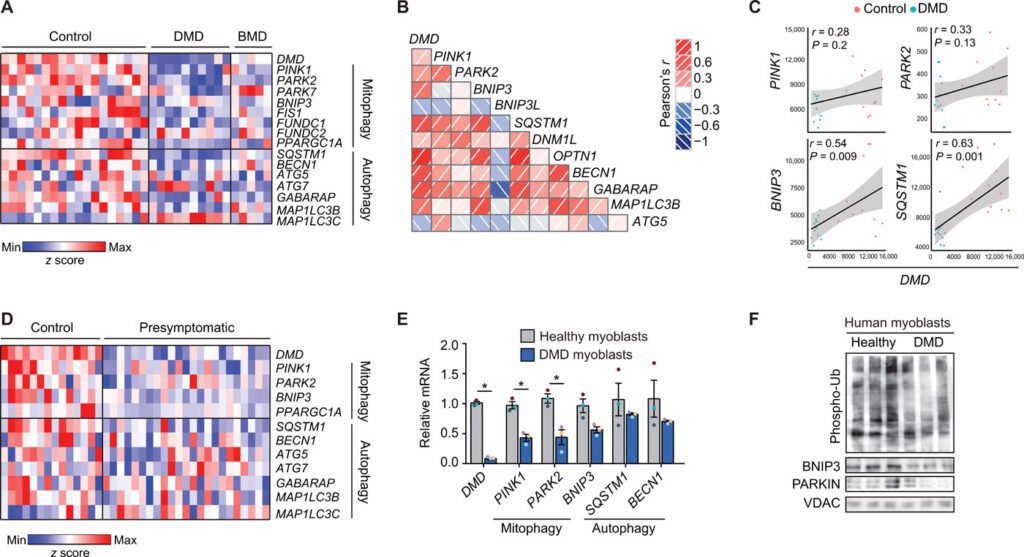
the role of mitophagy
In particular, gene expression models show that the development of Duchenne muscular dystrophy is associated with a marked decrease in mitophagy. Mitophagy is the process that cells rely on to remove and recycle defective mitochondria and keep energy levels high.
Duchenne muscular dystrophy is the most common fatal genetic disease diagnosed in childhood and there is no cure yet. Our work represents a significant breakthrough in the search for new therapeutic approaches for muscular dystrophies.
Johan Auwerx, MD, PhD, lead author and professor at EPFL.
Urolithin A and Duchenne muscular dystrophy
The natural compound urolithin A is known to activate mitophagy and improve mitochondrial health in both mice and humans. When the study scientists and lead authors, Peiling Luan e Davide D'Amico, they fed the compound to mice with Duchenne muscular dystrophy for just ten weeks. Levels of mitophagy effectively increased to bring them back to normal, with a significant reduction in muscle damage. THE mice with DMD given urolithin A, they saw a 45% increase in running performance compared to untreated control animals. And they lived longer: on average 40% more.
Importantly, for the human disease, urolithin A reduced a harmful condition called fibrosis in the heart and diaphragm muscles by 36% and 39%, respectively. Similar damage seen in patients with Duchenne muscular dystrophy typically leads to fatal cardiac or respiratory failure. Urolithin A was also able to improve the regeneration of mouse muscle stem cells. This is particularly relevant to the disease in humans since the onset of DMD is linked to the depletion of functional stem cells.
Prior to this study, it was understood that dramatic loss of muscle function in patients with Duchenne muscular dystrophy was associated with mitochondrial dysfunction. Here we found that defective mitophagy, the removal and recycling of dysfunctional mitochondria, play a key role in the progression of DMD.
Davide D'Amico, PhD, Project Leader at Amazentis and first author of the article